Product introduction
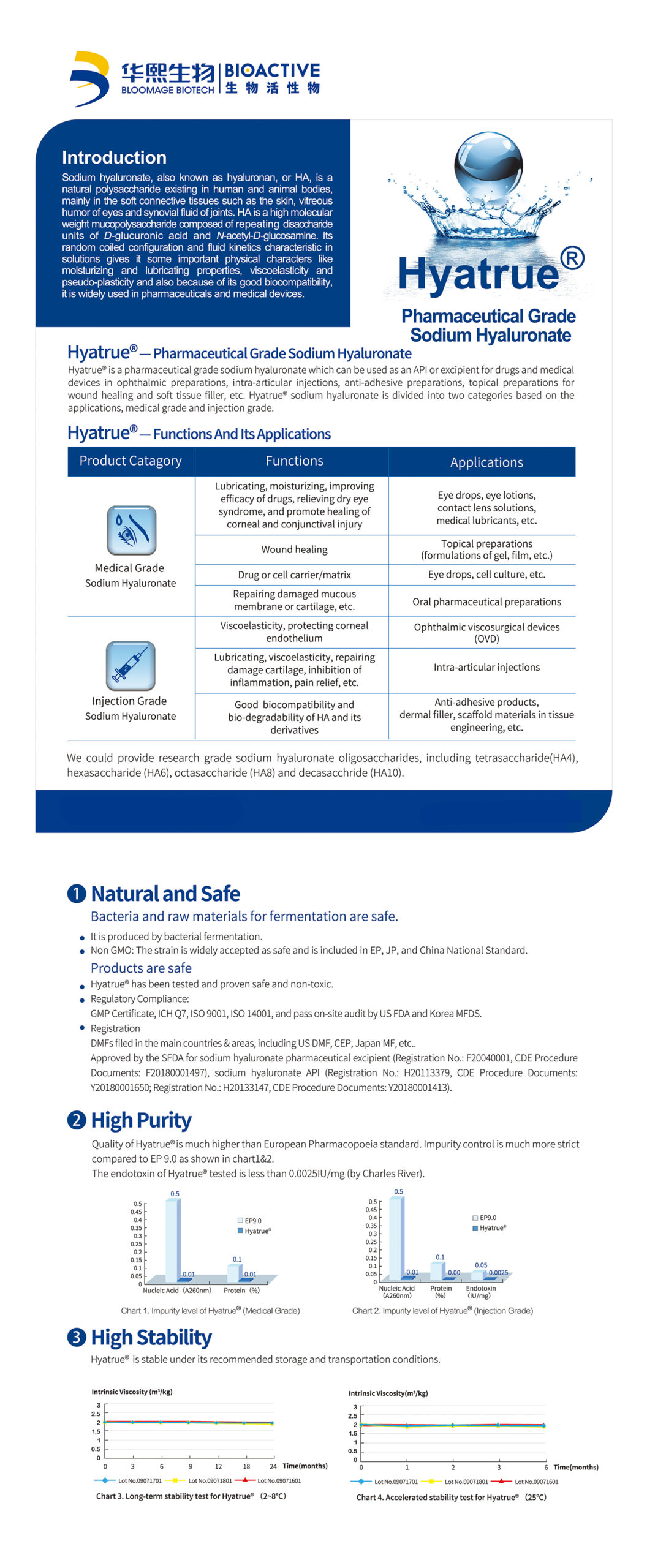
Sodium hyaluronate is a mucopolysaccharide composed of repeating disaccharide units of glucuronic acid and N-acetylglucosamine. It exists naturally in human and animal bodies. mainly distributed in joints, ocular vitreous, skin, and umbilical cord. Sodium hyaluronate has high hydrophilicity/moisture retention, viscoelasticity and lubricity. Hyatrue™ injection grade sodium hyaluronate is produced by fermentation and free of animal-derived materials. It is natural and safe with high purity and high stability.
Product application
For ophthalmic surgery; for knee arthritis, osteoarthritis and scapulohumeral periarthritis; for ophthalmic viscoelastic devices, dermal fillers, anti-adhesive agent, etc.
Product functions
Lubricating, moisturizing, viscoelasticity, support and filling, promotion of cartilage repair, anti-inflammation and pain relief. It has good biocompatibility and high biodegradability, etc.
Safety Standard
Produced naturally by Bacteria Fermentation; Non-GMO; EP, JP, China National Safety Standard
Purity
Much higher than EP Purity Standard. Impurity control is much more strict than EP11.0; The nucleic acid and protein impurity level tested is much lower than EP standard (by Charles River).
Stability
Stable under recommended storage and transportation conditions
Certification and standards
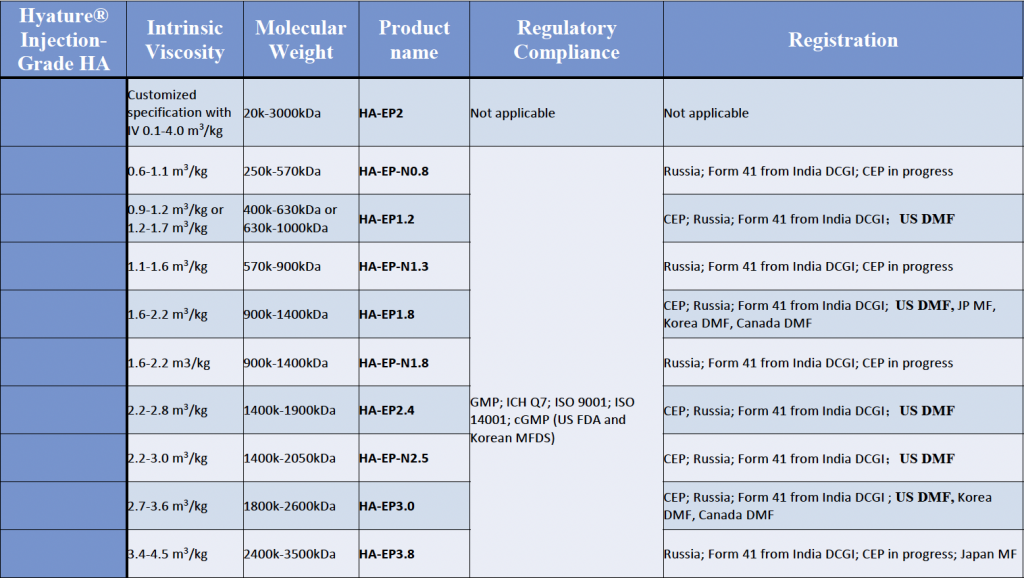
Certified by the China NMPA (status on CDE platform: A; material master file for dermal filler and injection aesthetics), US DMF, EU CEP, Korea KDMF, Japan MF and registration in Russia, India, and other countries or regions.
Physical form
Powder
Product specifications
50g/bottle, 100g/bottle, 200g/bottle, 1KG/bag
Precautions
Highly hygroscopic