Product introduction
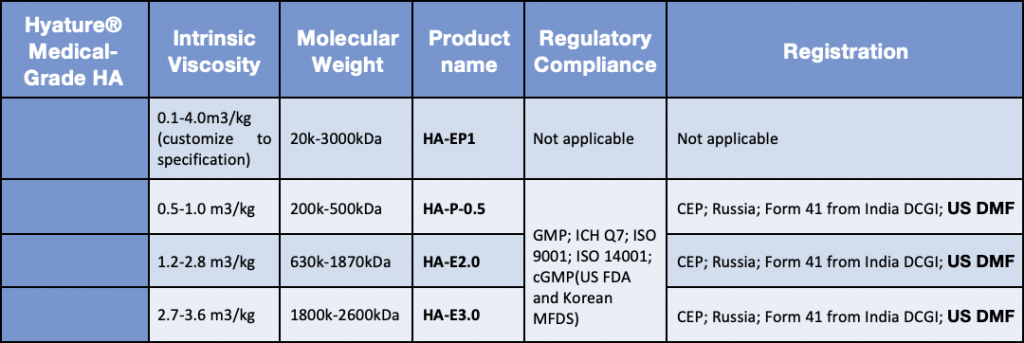
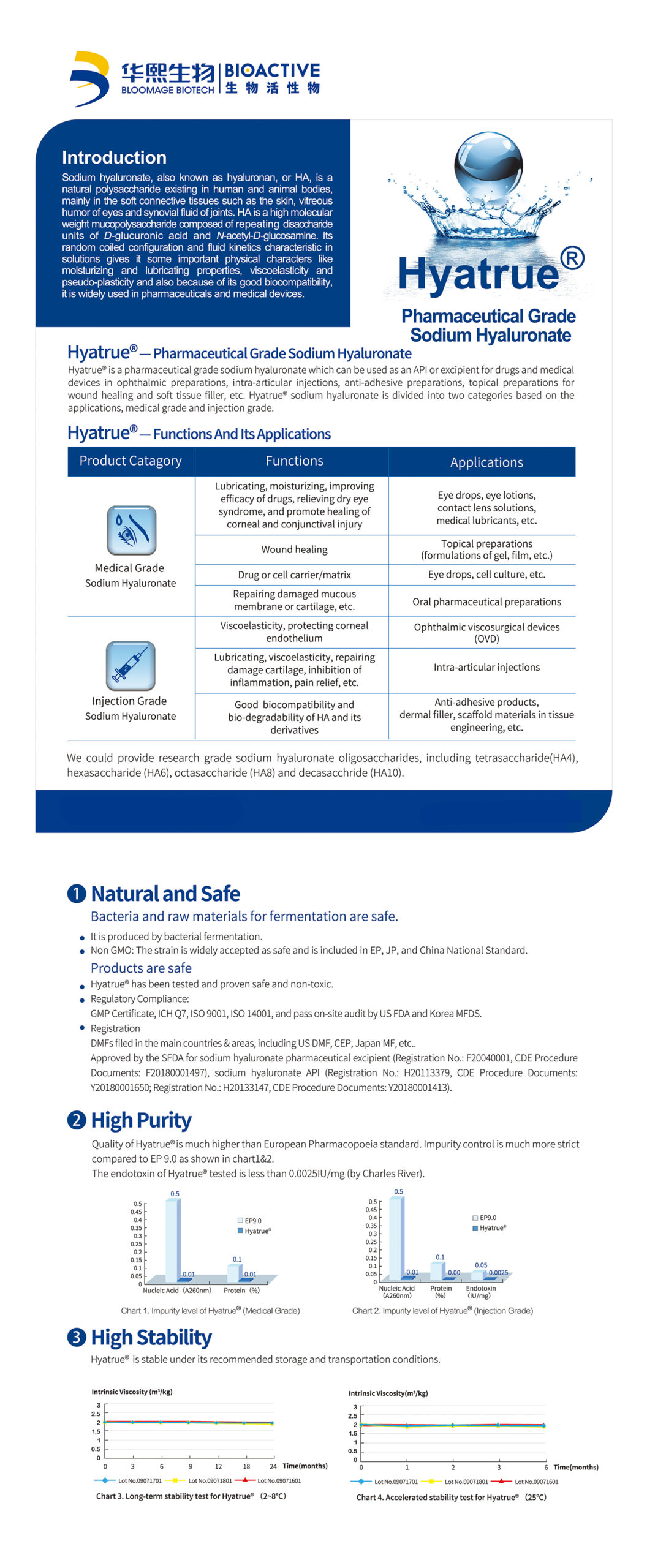
Sodium hyaluronate is a mucopolysaccharide composed of repeating disaccharide units of glucuronic acid and N-acetylglucosamine. It exists naturally in human and animal bodies. mainly distributed in joints, ocular vitreous, skin, and umbilical cord. Sodium hyaluronate has high hydrophilicity/moisture retention, viscoelasticity and lubricity. Hyatrue™ medical grade sodium hyaluronate is produced by fermentation and free of animal-derived materials. It is natural and safe with high purity and high stability.
Product application
Eye drops, lubricating solution, contact lens solution, topical preparations, oral pharmaceutical preparations, etc.
Product functions
Lubricating, moisturizing, improving efficacy of drugs, relieving dry eye syndrome, promoting healing of corneal and conjunctival injury, wound healing, repairing damaged mucous membrane, etc.
Safety Standard
Produced naturally by Bacteria Fermentation; Non-GMO; EP, JP, China National Safety Standard
Purity
Much higher than EP Purity Standard. Impurity control is much more strict than EP11.0; The nucleic acid and protein impurity level tested is much lower than EP standard (by Charles River).
Stability
Stable under recommended storage and transportation conditions
Certification and standards
Certified by the China NMPA (status on CDE platform: A), US DMF, EU CEP, Korea KDMF, Japan MF and registration in Russia, India, and other countries or regions.
Physical form
Powder
Product specifications
100g/bottle, 200g/bottle, 1KG/bag, 5KG/bag, 10KG/bag
Precautions
Highly Hygroscopic